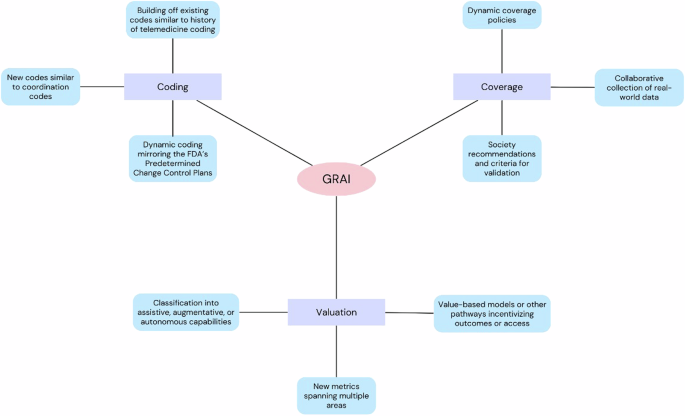
It remains to be seen how GRAI will be described and valued within the MPFS and other payment systems. Several questions will need to be answered, centered on three key areas: how will GRAI be coded, how will it be evaluated, and how will it be covered by other payers (Fig. 2) ?
Will GRAI receive its own CPT code?
There are currently two Category I CPT codes and six Category III codes. Each of them is specific to a targeted medical service, i.e. narrow AI. For example, CPT 75580 specifically addresses FFR imaging in the coronary circulation. In contrast, GRAI is more general and works across multiple activities and diverse clinical tasks.
A CPT code solution to describe GRAI may function more similarly to coordinating codes within the CPT rather than clinically specific radiology codes. CPT codes for care management describe coordinated activities that include and connect existing clinical services. For example, CPT 99491 describes chronic care management services provided by a physician that include establishing and following a comprehensive care plan.19. Such codes for GRAI can link different task types based on their usage (e.g., triage, diagnosis, reporting), as well as radiology modalities and subspecialties.
For example, a single multitask coordination code could cover multiple tasks in a study, such as triaging a chest CT scan based on suspected pathology, detecting and segmenting anomalies, and generating structured reports. Such codes could be applied to several GRAI applications and types. Alternatively, a series of modality-specific codes could be developed with separate codes for the aforementioned levels of GRAI tasks, which could allow for more granular differentiation, for example if a brain MRI GRAI tool was used solely for detection or for the complete pipeline from triage to reporting. A suite of codes could better align with the current reimbursement structure, where complexity and terms impact assessment.
More broadly, CPT coding for GRAI may parallel the history of telemedicine coding, which first reflected the idea of telemedicine as an extension of existing in-person care visits before the development of codes and of modifiers specific to telemedicine.20. GRAI coding could also start by building on existing radiology codes before having its own comprehensive framework. For example, new modifiers for brain MRI would indicate the level of GRAI involved, again ranging from detection/diagnosis to reporting. This strategy can scale more easily and avoid creating an overwhelming number of specific codes.
Another important consideration is that GRAI is by nature an evolving technology; by definition, it must be able to adapt to newly described tasks. Traditional CPT codes are designed for static technologies whereas GRAI will require a more adaptive strategy. The FDA faces similar challenges with AI/ML devices and has proposed predetermined change control plans (PCCPs) that will allow pre-approved modifications to these devices without the need for full reapproval.21. CMS should take a close look at how FDA implements PCCPs, as similar strategies could help guide GRAI coding.
If so, how will it be valued?
RBRVS is a system based on relativity. Thus, the new GRAI codes will be compared to other AI codes, particularly those in radiology. Given the comprehensive nature of the GRAI and the ability to apply it to several different tasks, will it be more valued in the RBRVS? For example, depending on the specific task and needs of a given user, GRAI could be used for assistive purposes (e.g. detecting and highlighting abnormalities in an imaging study for the radiologist), increase (for example, long-term prognosis). based on these anomalies), or autonomous capabilities (e.g., generating comprehensive reports including differential diagnoses and recommendations).
As GRAI applications become more autonomous, the complexity of its contributions increases and should be reflected in its valuation. Furthermore, GRAI can be applied in many areas and its valuation must therefore be based on measures that also cover several areas leading to a composite and multifaceted RVU. For example, a composite GRAI RVU could take into account the radiologist’s work required for a given task, may include a higher task complexity component for stand-alone tasks such as reporting, and an adaptability component for tools integrating multimodal data. It is likely that more AI/ML codes will be evaluated and given RVUs before GRAI, so these precedents will be relevant.
Continued adoption of AI tools may also lead to changes in what we value and the underlying metrics that will affect GRAI’s valuation. Many authors have suggested alternative reimbursement pathways to free models, such as outcomes or access incentives.12,22. Others have noted that value-based models might better address circumstances where the functions of AI tools cannot be appropriately divided into separate services, which would certainly apply to many applications of GRAI .23.
What other steps will be needed for coverage and dissemination in patient care?
Obtaining a CPT code and even an RVU valuation does not guarantee adoption. Coverage decisions will be critical in determining whether GRAI will be reimbursed by Medicare and other payers. Medicare coverage is influenced by National Coverage Determinations (NCDs) that apply to particular services or technologies throughout the United States, as well as Local Coverage Determinations (LCDs) set by Medicare administrative contractors. Medicare (MAC) that process Medicare claims in specific regions. Meanwhile, private payers have their own criteria, which often, but not always, match those of Medicare.
Although these criteria vary, for example between payers or MACs, a number of universal elements are considered, such as the demonstrated effectiveness and safety of a tool, comparative effectiveness against standards of existing care, cost-effectiveness and utilization. All of these will require the collection of real-world data that investigate GRAI in a wide range of clinical settings and applied to diverse patient populations. Indeed, voices are already being raised to call for more randomized trials on AI tools.24. Collecting this data will likely be more difficult for GRAI than for existing AI technologies, given that they can be deployed in many more versatile situations; developers, clinicians, and payers must collaboratively plan how to collect the necessary real-world data. Additionally, just as CPT codes are designed to work with static technology, coverage policies are currently not well equipped to handle dynamic tools and need to be revamped in anticipation of the emergence of GRAI. Because the society’s recommendations are also crucial to ensuring broad coverage, the ACR and other radiology organizations should consider how best to validate the GRAI.
The literature requirements to obtain coverage are similar to those needed to obtain a CPT code, but may also be different. For example, a greater focus on results and technical considerations may apply.